Batteries actively power modern electronics, driving devices from smartphones and tablets to drones and electric vehicles (EVs). The global battery market actively grows, driven by advancements in EVs and the proliferation of portable electronics, reaching a value of approximately USD 113.4 billion. Batteries are categorized into two main types: primary (non-rechargeable) and secondary (rechargeable). Understanding these battery types is essential for selecting the right one for specific use cases. By doing so, users can optimize device performance, efficiency, and lifespan. Active selection enables informed decision-making.
Types of Batteries:
(a) Primary Batteries
Primary batteries are disposable and cannot be recharged once depleted. They are designed for single-use applications, where recharging is unnecessary or impractical.
1. Alkaline Batteries
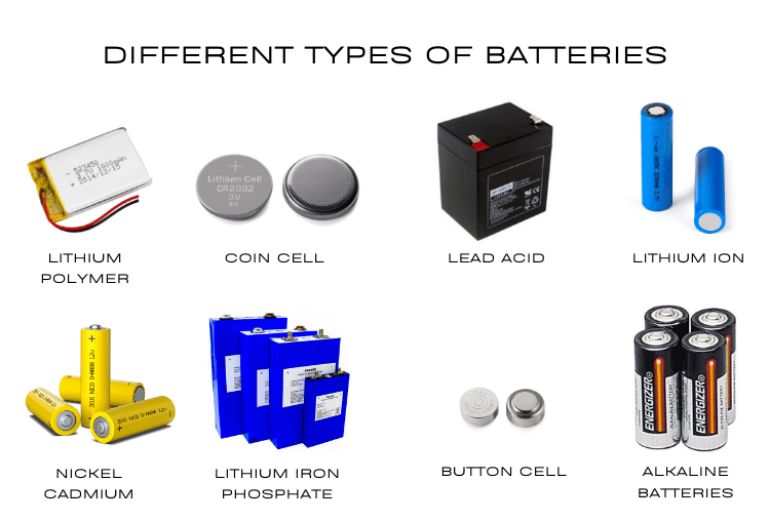
Alkaline batteries are one of the most common types of primary batteries. They use a chemical reaction between zinc and manganese dioxide and are named for the alkaline electrolyte, usually potassium hydroxide. These batteries are highly efficient, have a long shelf life, and are small in size, making them ideal for low-drain devices like remote controls, clocks, and flashlights. Available in sizes such as AA, AAA, and 9V, alkaline batteries are widely used in household devices. However, they are less suitable for high-drain devices like digital cameras. Proper recycling is important due to environmental concerns.
2. Aluminum-Air Batteries
These batteries offer the highest energy density and generate energy through a reaction between aluminum and oxygen. They are used in specialized applications but are non-rechargeable, making them suitable for single-use applications. Once the aluminum is exhausted, the battery must be disposed of.
3. Dry Cells
Dry cells are another type of primary battery. They use a dry electrolyte rather than a liquid one. Although these batteries are still used in some low-power devices, they are being replaced by more efficient alkaline batteries. Dry cells were once common in toys and remote controls.
(b) Secondary Batteries
Secondary batteries, or rechargeable batteries, can be used multiple times, offering the advantage of recharging and reusing them across numerous cycles. These batteries are more commonly found in high-drain devices like EVs, smartphones, and portable gadgets.
1. Lithium-Ion (Li-Ion) Batteries
Lithium-ion batteries are among the most widely used secondary batteries due to their high energy density and relatively long lifespan. These batteries use lithium salts and a liquid electrolyte to move ions between the cathode and anode. Li-Ion batteries are found in devices such as smartphones, laptops, tablets, and electric vehicles. They are lightweight, compact, and capable of enduring hundreds of charge cycles before showing significant degradation. Despite these advantages, Li-Ion batteries require proper care to avoid safety risks, such as overheating or catching fire when damaged or charged incorrectly.
2. Lithium-Polymer (Li-Po) Batteries
Li-Po batteries are similar to Li-Ion batteries but use a polymer electrolyte instead of a liquid one. This makes them lighter and more flexible, which is beneficial for applications requiring compact or custom-shaped batteries, such as drones. Li-Po batteries offer a higher energy density (185Wh/kg) compared to Li-Ion batteries (126Wh/kg), but they are more expensive and may have slightly lower performance over time. Due to their light weight and high energy density, Li-Po batteries are ideal for drones and other portable electronics.
3. Nickel-Metal Hydride (NiMH) Batteries
NiMH batteries are an improvement over older Nickel-Cadmium batteries, using a hydrogen-absorbing alloy instead of cadmium, making them more environmentally friendly. These batteries have a higher energy density than Nickel-Cadmium batteries and are less prone to the memory effect, where partial discharges reduce the battery’s capacity. NiMH batteries are commonly used in household electronics like digital cameras and in hybrid vehicles. However, they do suffer from higher self-discharge rates, meaning they can lose charge faster when not in use. The power density of NiMH batteries is approximately 100Wh/kg.
4. Lead-Acid Batteries
Lead-acid batteries are one of the oldest types of rechargeable batteries. They use lead plates and sulfuric acid as the electrolyte and are known for their low cost and high power output. These batteries are often used in automobiles to start engines and power electrical systems, as well as in uninterruptible power supplies (UPS) and grid storage. However, lead-acid batteries are bulky and have a lower energy density compared to modern alternatives like Li-Ion batteries. They also degrade over time due to sulfation, which limits their lifespan. Despite their drawbacks, lead-acid batteries are still commonly used where cost is a major factor.
5. Solid-State Batteries
Solid-state batteries are an emerging technology with the potential to surpass conventional lithium-ion batteries in terms of safety, energy density, and lifespan. Unlike Li-Ion batteries, which use a liquid electrolyte, solid-state batteries use a solid electrolyte. This eliminates the risk of leakage and improves safety by reducing the likelihood of fires or explosions. Although solid-state batteries are still in development and not yet widely available, they hold promise for applications in electric vehicles, consumer electronics, and other industries. Once commercialized, they could offer more efficient and safer energy storage solutions.
6. Zinc-Carbon Batteries
Zinc-carbon batteries are a primary battery type, commonly used in low-drain devices like flashlights and toys. These batteries are inexpensive but offer lower energy output and a shorter lifespan than alkaline or lithium batteries. Zinc-carbon batteries are made with a zinc anode and a carbon cathode, and an electrolyte of ammonium chloride. Although they are cost-effective, they are less efficient, more prone to leakage, and have a higher self-discharge rate. As such, they are generally being replaced by more efficient and longer-lasting alternatives.
7. Sodium-Ion Batteries
Sodium-ion batteries are an emerging alternative to lithium-ion batteries. They use sodium ions instead of lithium ions to carry charge, making them more abundant and less expensive. Sodium-ion batteries currently have a lower energy density compared to lithium-ion batteries but hold promise for large-scale applications like grid storage and electric vehicles due to their cost and sustainability advantages. Research is ongoing to improve the performance of sodium-ion batteries, and they may become a viable alternative in the future.
8. Flow Batteries
Flow batteries are a type of rechargeable battery where energy is stored in liquid electrolytes that flow through the system. The chemical reactions that generate electricity occur in the electrolyte, which is stored in external tanks and pumped through the system. Flow batteries are ideal for large-scale applications such as renewable energy storage. They offer long-duration energy storage, high durability, and less degradation over time. However, they are still in the early stages of development and are not widely used in commercial applications yet.
Conclusion
Batteries actively power a wide array of devices, and their types vary according to energy needs, usage scenarios, and environmental conditions. Primary batteries, such as alkaline and dry cells, actively cater to single-use applications. Meanwhile, secondary batteries like lithium-ion, lithium-polymer, and lead-acid batteries actively recharge and power devices requiring frequent charging.
Simultaneously, emerging technologies like solid-state and sodium-ion batteries actively enhance battery performance and sustainability. Consequently, as battery technology continues to evolve, new solutions actively emerge to meet the growing demand for energy storage across various industries.